Vickor Medical "Arkcess® Left Ventricular Wire" approved NMPA registration Certificate!
Time:
2023-12-29
On December 26, the "Left ventricular guide wire" independently developed by VECO Medical obtained the registration certificate of the National Medical Products Administration (NMPA) (Registration certificate number: National Instrument Registration Permit 20233032030). This product is suitable for diagnostic and therapeutic procedures performed via aortic vascular intervention in the left ventricle.
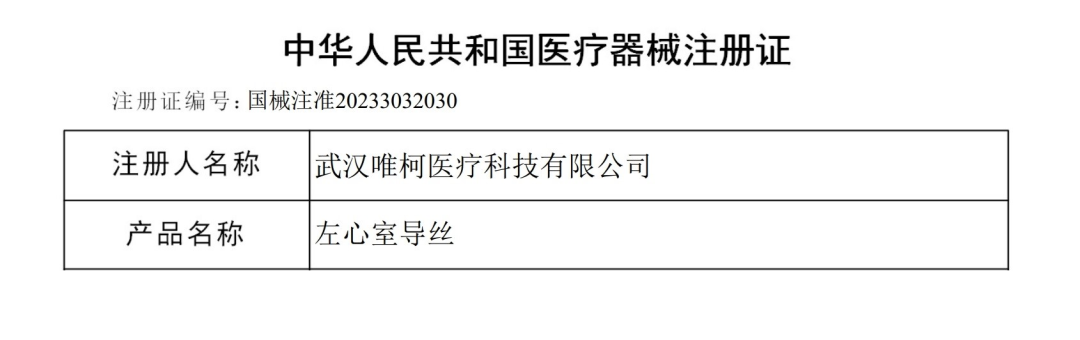
The Arkcess® left ventricular guide wire consists of stainless steel core wire, wound wire, coated with a hydrophobic coating, and pre-molded head end. It has the characteristics of strong support, clear development, safe guidance and so on to improve the efficiency of surgery.




©2023 Wuhan Vickor Medical Technology Co., Ltd.
This site already supports IPV6 access | SEO | Business License

